Calories?!? Oy vey, who wants to hear that topic, especially this time of year? Well, as you sit around the fireplace, you can think about the heat being given off by the firewood, and understand it a bit better (and as you enjoy some black garlic, nosh on some healthy dried fruit, or feed your doggie my treats :-p )
How did I pick "enthalpy" as a topic? Well, like most of my mental meanderings, this email might best be re-titled as: “The Solo-Scientist Socratic Method”.... aka: Talking to yourself.
The great thing about the internet is you can go down rabbit holes super quickly (for better or for worse!) I was curious why acetylene gas was better for welding than methane, beyond the simple answer: “it burns hotter.” Why does acetylene burn hotter? My questioning might be eruditely/optimistically described as “the Socratic method”, although most folks today just call this “nerding out.” That’s when I arrived at “Bond Enthalpies.”
We were taught bond enthalpies in high school and if you were really lucky, you took college chemistry! Oy vey #2. So here I am, bringing the topic of BOND ENTHALPY back to you today! (The last thing, nay, the unknown unknown thing you thought you’d be reading about right now!)
Jumping right in, here’s a table of bond enthalpies: 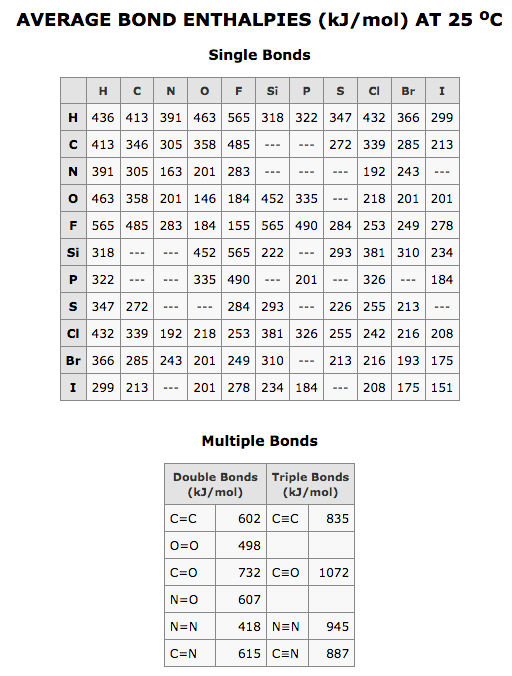 Looks like just a bunch of numbers? Nay, this is a very special table. This table was super-hard to find (I’m not sure how I found it the first time, so credit to the chemistry department at the University of Massachusetts who posted it.) I like this table a lot more than all the other bond enthalpy tables because it’s a matrix, rather than a list, so a lot more information can be conveyed in the same space, and yet it’s still very intuitive once you understand the basic concept of bond enthalpy.
Looks like just a bunch of numbers? Nay, this is a very special table. This table was super-hard to find (I’m not sure how I found it the first time, so credit to the chemistry department at the University of Massachusetts who posted it.) I like this table a lot more than all the other bond enthalpy tables because it’s a matrix, rather than a list, so a lot more information can be conveyed in the same space, and yet it’s still very intuitive once you understand the basic concept of bond enthalpy.
And what makes this table so interesting? Well, it applies not just to my world of food science, but it also explains all matter/molecules, even the challenge of global warming!
First: what is enthalpy?
Enthalpy is the heat energy to BREAK a chemical bond. (hence why it’s related to my initial question of methane vs acetylene, but also all food molecules contain chemical bonds in which we derive energy.) A higher value, means a stronger bond. Enthalpy is often taught in combination with the concept of “internal energy” and Pressure x Volume, but then it gets a bit confusing and beyond this scope. I’ve also read these values might only apply to gases, so a proper chemistry professor can likely do a better (or worse) job of confusing you (and me.)
Enthalpy is additionally a bit confusing because higher bond enthalpy means it takes more energy to break a bond, but it doesn't necessarily mean there's more energy, since the total molecule and reaction (reactants and products) need to be fully added up, and the difference taken between the before (reactants) and after (products.) Regardless, the above table shows how double and triple are harder to break than single bonds. Take a C=O double bond. It's a very strong bond at 732kJ/mol: two of these bonds are found in Carbon Dioxide. Hence why CO2 is hard to get out of the atmosphere (and needs to be done by photosynthetic organisms with sunlight.)
Look at O=O (oxygen: O2.) It’s bond enthlapy is just 498 kj/mol. And it's about 20% of what we breath: O2 is quite a reactive molecule! (hence why it causes rusting, both of metal, and our DNA.) The other 80% of air is Nitrogen gas, N2, but it is triple bonded. It has a super-strong bond enthalpy of 945 kJ/mol, hence why it's so inert.
In any chemical reaction, if you add up the bond energies between each atom in the reactants, and then the products, if the final products have more bond energy, then energy is released as heat in the reaction, and the exact amount of heat can be calculated! This energy released is called “bond dissociation energy.” (Sometimes we experience this in unfortunate relationships?) This bond dissociation energy can even be measured for food in a "calorimeter."
Here's a nice visualization of the combustion of methane (natural gas):
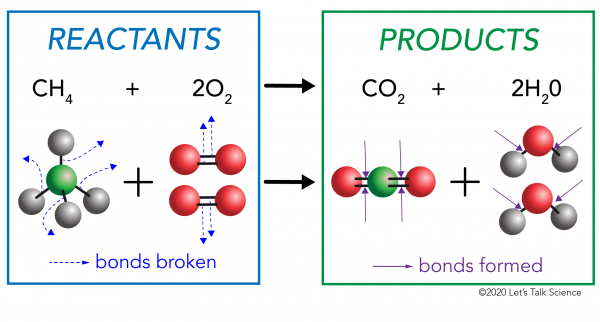 To answer my own question about why acetylene is a "hotter" gas, without showing all the math, acetylene releases twice the CO2 (since it has a Carbon-Carbon triple bond. CO2 is composed of a very stable, high bond-energy (C=O)*2, and acetylene also produces half the water vapor, which might even have a cooling effect on the flame? The acetylene combustion reaction balances out to consume 25% more oxygen than a methane combustion, which is why in practice, plumbers use an oxygen tank with acetylene. (Tell that to your plumber! See what reaction you get!)
To answer my own question about why acetylene is a "hotter" gas, without showing all the math, acetylene releases twice the CO2 (since it has a Carbon-Carbon triple bond. CO2 is composed of a very stable, high bond-energy (C=O)*2, and acetylene also produces half the water vapor, which might even have a cooling effect on the flame? The acetylene combustion reaction balances out to consume 25% more oxygen than a methane combustion, which is why in practice, plumbers use an oxygen tank with acetylene. (Tell that to your plumber! See what reaction you get!)
I'd go further into another example of comparing:
1. Glucose respiration reaction vs.
2. Photosynthesis reaction (the reverse of #1 glucose-respiration)
But... this email is already too long, so I'll save it for another time.
Just take-away one additional thing: the definition of a "calorie" is the heat needed to raise 1 gram of water by 1 degree Celsius (so we're all just a bunch of walking hot-water heaters?)
(1 calorie is the same as about 4.2 joules; On a nutrition facts food-label, a "calorie" is actually 1000 calories, which is 1 "kcal" ~ 4200 Joules, but in English vernacular, we call a kilocalorie a “calorie.” This is important if you want to do some calculations on your own using your new enthalpy knowledge, for those folks out there who (hopefully!) decide to go down a food chemistry-curiosity rabbit-hole!)
ii. New batch of BLACK GARLIC!
(amazingly delicious AND nutritious!)
Farmers Market special: 2nd garlic item, $1 off!
iii. New batch of K9 Bros Chicken!
Bring your doggie to the market!
Bubble Wand stocking-stuffer!
2. When to see moi next?
Answer: This weekend!
Here's my full winter/indoors schedule:
|
Saturdays |
Sundays |
|
| This weekend! |
N/A |
Dec 11th
|
| Next time: |
Jan 14 |
Jan 15 |
|
Bi-weekly thereafter (every 2 weeks) |
Jan 28 Feb 11 Feb 25 Mar 11 Mar 25 |
Jan 29 Feb 12 Feb 26 Mar 12 Mar 26 |
More info & directions:
Glen Cove-Deep Roots Farmers Market Facebook Page
Huntington Farmers Market Directions

3. Deals!
Subscription direct to your door!
If you can’t come to my products, make my products come to you!
Subscribe and Save! 5% off and free shipping on qualifying orders!
Shameless plugs
Fresh dried fruit is a weight loss cure!
(this statement that has not been substantiated by any scientific study... but no sugar added dried apples and pears can't be bad!)
No sugar added! USA grown! New York State Red Apples! Slow and low dehydrated to preserve vitamins, antioxidants, and flavors!
(compare to other brands apple chips which are high-temperature baked)
1) Red Apples!
2) Green Apples!
3) Pears!
4) Nectarines!
5) Hmmm... what's next?

Conclusion:
Hypothesis: I will see you this Sunday/tomorrow! 😃
9:00am to 1pm!
423 Park Avenue, John J Flanagan Center
Null hypothesis: I will not see you this weekend 😭
Conclusion: Not sure! Let's see!
Website coupon: 10% off black garlic with coupon code "blackgarlic"
![]()
★ k9bros.com ★
☆ gourmet-magic ☆
★ bubble-science ★











.png)